INCENTIVE
Identification of persistent neurological complications after eclampsia and discovery of novel neuroprotective treatments to improve maternal outcomesThe goal of this project is to define neurological outcomes after eclampsia and to determine underlying
pathophysiological pathways to eclampsia which will lead to new drugs for neuroprotection of the
maternal brain.
Preeclampsia and eclampsia are the most common causes of direct maternal death globally.
Magnesium sulphate is the only treatment in use, but it has serious side effects, only protects from
seizures in 50% of cases and neuroprotection on longterm is not established. Women with eclampsia
run an increased risk of longterm neurological sequelae but causality with the acute insult is not
proven. There is an urgent need to understand this relationship and underlying pathophysiological
pathways to identify how the acute injury affects the chronic sequelae and identify new targets for
neuroprotective drugs.
We have recently shown that acute neurological complications in eclampsia may be caused by a
disturbed regulation of blood flow to the brain in combination with an injured blood-brain barrier that
leads to cerebral edema, neuroinflammation and decreased seizure threshold (Bergman et al, 2021;
Friis et al, 2022). Based on these findings, we hypothesize that acute brain injuries in preeclampsia and
eclampsia do not completely recover after delivery and may partly persist six to twelve months after
delivery, causing severe outcomes for the mother such as impaired cognitive function, neurological
deficits and persistent brain injury that can be reversed by neuroprotective treatment after the acute
insult. These outcomes can be detected by poor performance on objective and subjective cognitive
function tests, deficits in neurological examination and impaired dynamic cerebral autoregulation with
persistent lesions on MRI brain.
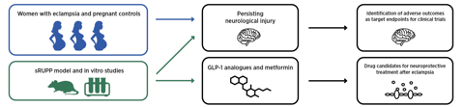
To test our hypothesis, we will use results from clinical investigations in women with eclampsia
together with pre-clinical models to determine underlying pathophysiological processes and the
recovery from neurological injury after delivery. In addition, we will trial neuroprotective treatment in
an animal model to identify new promising drugs for neuroprotection in preeclampsia and eclampsia.
We will make use of a cohort of women with eclampsia from our site at Stellenbosch University,
South Africa, as well as a rat model of preeclampsia and an in vitro model of preeclampsia in our lab
at the University of Gothenburg, Sweden, to evaluate these outcomes. We will use anti-diabetic and
anti-epileptic drugs commonly used in pregnancy and test these in rat model of preeclampsia to find
new neuroprotective treatments that improve maternal outcomes.
This could enable us to protect the maternal brain, save lives and reduce morbidity globally.
The specific aims include:
Specific Aim I: To characterize persisting neurological injury in women with eclampsia 6-12 months postpartum. We will measure dynamic cerebral autoregulation, evidence of blood-brain barrier disruption, glial and axonal cell injury, MRI lesions and cognitive function 6-12 months after eclampsia.
Specific Aim II: To determine molecular mechanisms underlying acute neurological injury in preeclampsia and eclampsia. A clinical cohort, and a rat model of preeclampsia will be used as well as in vitro models of the blood brain barrier and neuroimmune cells.
Specific Aim III: To determine the effects and mechanisms for neuroprotection in preeclampsia after administration of magnesium sulphate, metformin, Keppra and GLP-1 analogues. We will use targets previously identified by us and new targets identified in Specific Aim II to select the most promising neuroprotective treatments in preeclampsia.
The project is funded by the European Research Council Starting Grant and will start during the spring 2025.
