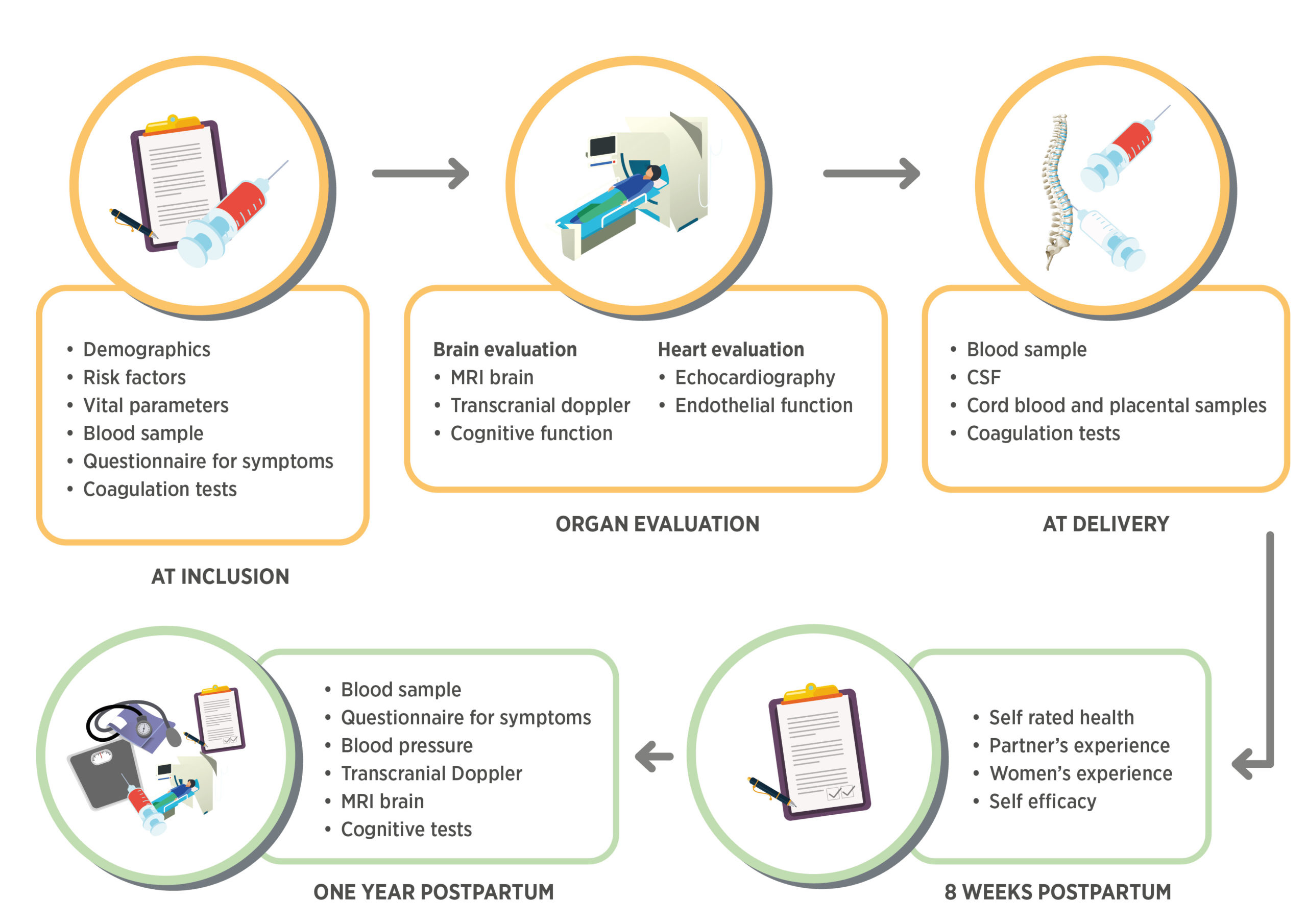
GO PROVE and UPMOST are parts of a prospective multicenter cohort study for investigating organ manifestations of preeclampsia. Pregnant women ≥18 years, diagnosed with preeclampsia presenting at Sahlgrenska University Hospital, Uppsala University Hospital or Södra Älvsborgs Hospital, Sweden, are included. Women ≥18 years with healthy pregnancies are included as controls. The study involves bio samples stored in a biobank, brain magnetic resonance imaging, cerebral doppler, echocardiography, peripheral artery resistance measurements, neurocognitive assessment and questionnaires and interviews for women’s and partner’s experiences. Participating women will also be scheduled for a one year postpartum follow-up investigation with additional blood samples and physiological investigations such as brain magnetic resonance imaging, cerebral doppler and cognitive function scoring.
By creating a Swedish data- and biobank for preeclampsia, we will provide the means to explore the disorder in a much broader sense and allow clinical and laboratory discoveries that can be translated to clinical trials aiming at improved patient centered care of women with preeclampsia. Further, to evaluate experiences and psychological impacts of being affected by preeclampsia can improve the care of both the women and their partners.
Trial Registration
Trial ID: ISRCTN13060768
Date registered: 27/07/2020
Link: https://www.isrctn.com/ISRCTN13060768
GO PROVE Participant’s Information