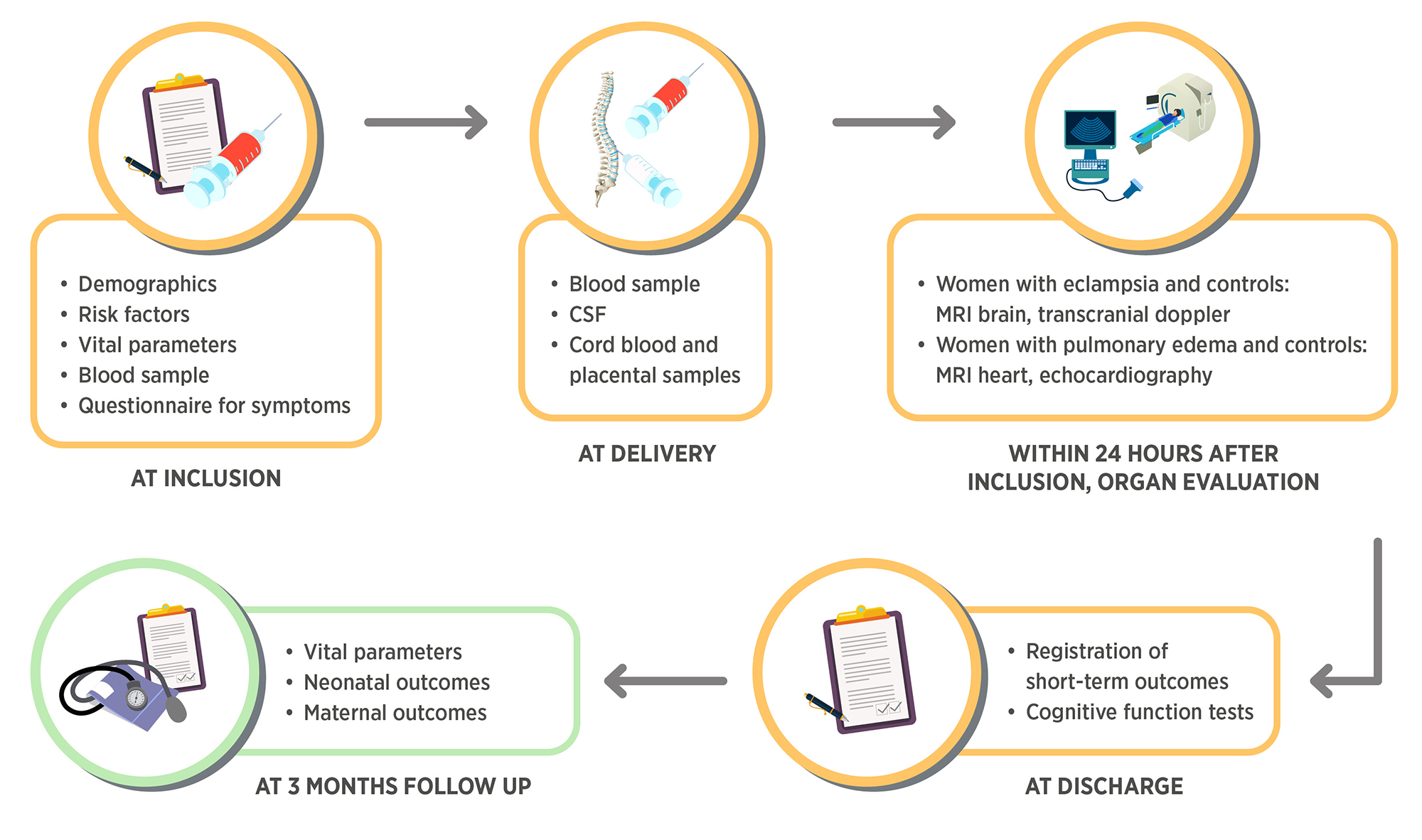
We have developed a preeclampsia biobank which we have conservatively designed with respect to allaying any potential ethical concerns. The approach of our biobank is:
- To collect biological specimens including plasma, saliva, cerebrospinal fluid, urine, placenta and umbilical cord blood in order to analyse potential biomarkers for organ injury in women with preeclampsia and organ complications
- To collect detailed data according to international consensus of core predictors and outcomes in preeclampsia research to analyse possible predictors and diagnostic tools for organ complications in preeclampsia
- To add tests and biophysical examinations to confirm or rule out organ dysfunction including magnetic resonance (MRI) brain, MRI heart, transcranial doppler, echocardiography and cognitive function tests in order to reveal underlying pathophysiological pathways and relate the findings to above predictors and biomarkers
Women with preeclampsia and women with normotensive pregnancies are enrolled in the biobank at admission to Tygerberg University Hospital for delivery or at time of a diagnosis of preeclampsia. Biological samples are collected at inclusion/delivery for all included women and during the hospital stay for women admitted for preeclampsia. Samples are stored in a -70 degree freezer until analysis. Background characteristics, clinical data and vital parameters are recorded throughout the hospital stay. At discharge and at 2 months follow up, cognitive function is assessed and additional outcome variables are collected. All data is stored in an online database. Women with eclampsia and corresponding controls are offered to undergo magnetic resonance imaging of the brain and doppler examination of cerebral bloodflow. Women with pulmonary edema and corresponding controls are offered to undergo magnetic resonance imaging of the heart and echocardiography.
This project aims to establish a biobank and database for severe organ complications to preeclampsia. This is only possible in a low-middle income country where the incidence of complications is high. The study integrates different methods and engages clinical and pre-clinical researchers to investigate eclampsia and organ complications with a focus on improved understanding of pathophysiology, possibility for prediction and in the long run hopefully drug evaluation and drug discovery.
Trial registration details
Title: A study for investigating organ complications in preeclampsia
Trial ID: ISRCTN10623443
Date registered: 16/07/2020
Publications from PROVE